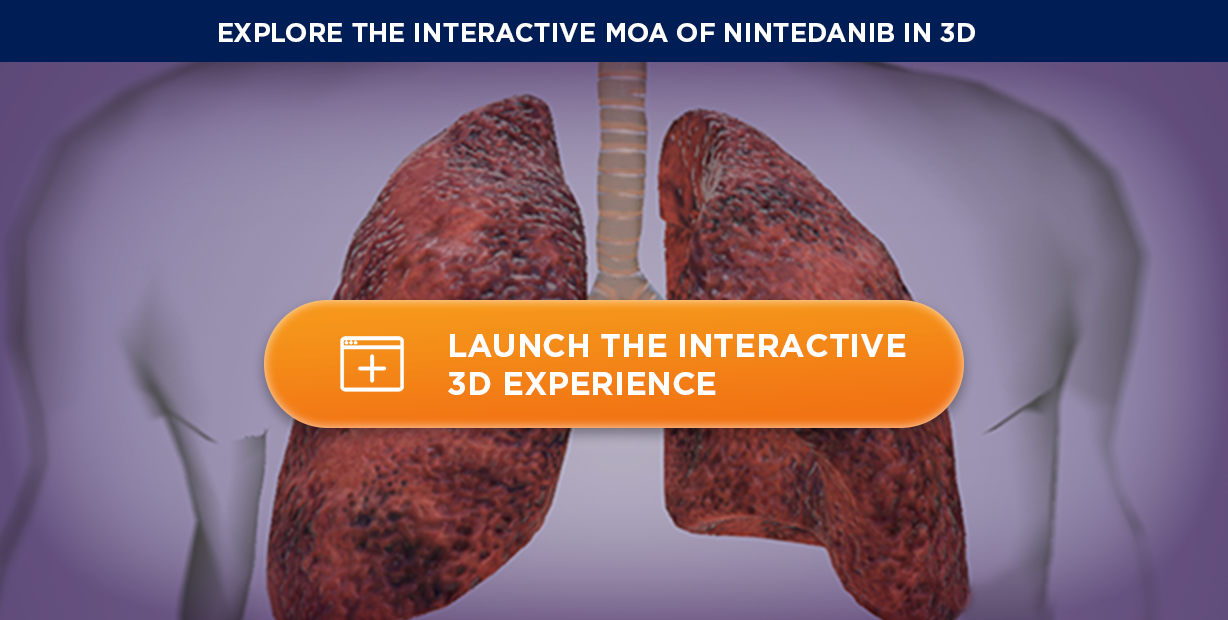
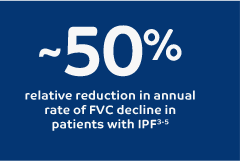
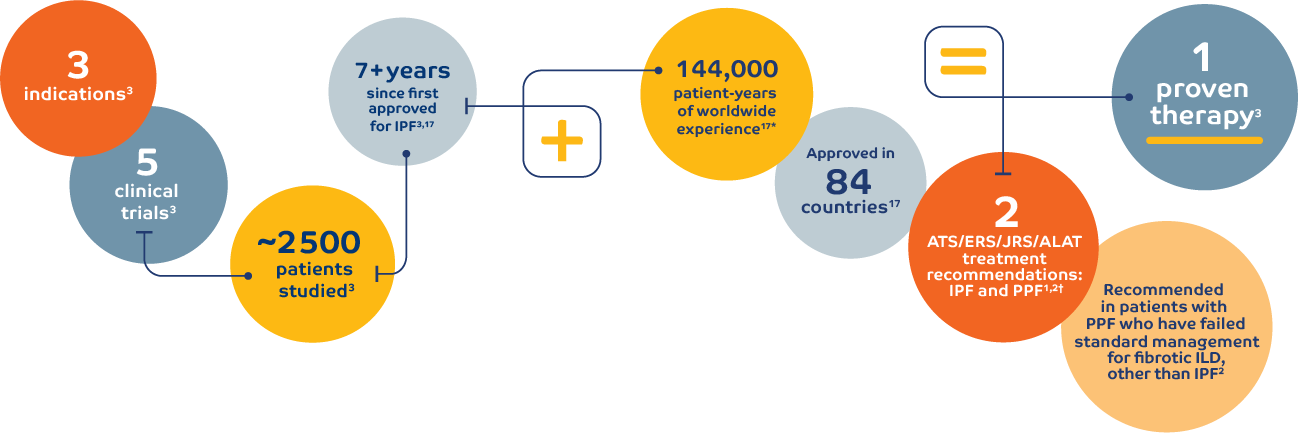
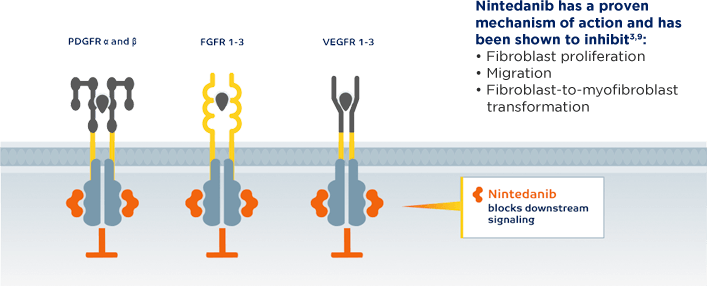
Recommended in patients with PPF who have failed standard management for fibrotic ILD, other than IPF2
OFEV Is Making Progress
Against Progression

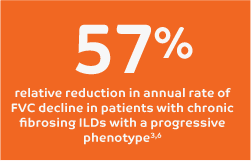
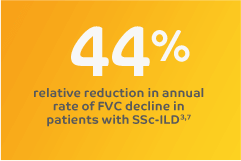
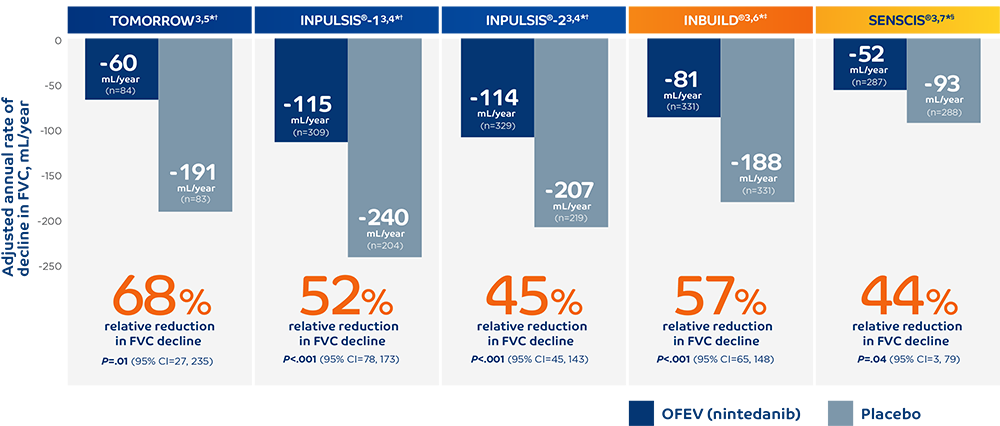
With consistent results across 5 clinical trials, OFEV (nintedanib)
is advancing the management of fibrosing ILDs.
Scroll to learn more about the clinical data, mechanism of action,
and worldwide experience of OFEV3-7
FOLLOW the PATH.
SCROLL TO SEE OUR PROGRESS